Understanding the stability of mixed A-cation lead iodide perovskites†
Abstract
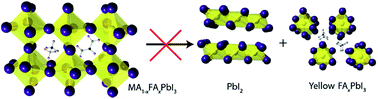
The routes and kinetics of the degradation of thin films of methylammonium (MA)/formamidinium (FA) lead iodide perovskites (MA1−xFAxPbI3, 0 ≤ x ≤ 1) under dry atmospheric conditions have been investigated. MA-rich phases decompose to the precursor iodide salts and PbI2, while FA-rich phases convert mainly to the yellow hexagonal phase. The reactivity is strongly inhibited for mixed cation phases of MA1−xFAxPbI3, for x = 0.4 to 0.6, where the decomposition routes available to end member phases become less favourable. It is shown that for pristine films with x = 0.6, PbI2 formation can be completely suppressed for up to 10 days. Kinetic analysis reveals that the rate of PbI2 formation decays exponentially with increasing FA content until x = 0.7, beyond which the FA containing perovskite transforms rapidly to the hexagonal phase. Ab initio simulations of the decomposition reaction energies fully support the increased kinetic stability found experimentally for the mixed A-cation perovskites.


 Please wait while we load your content...
Please wait while we load your content...