An advanced elastomer with an unprecedented combination of excellent mechanical properties and high self-healing capability†
Abstract
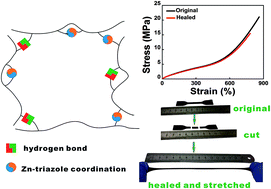
Rubbers are widely applied in tires, seals, biomedical materials and aerospace applications because of their unique high elasticity. However, combining high self-healing capability and excellent mechanical performance in a rubber remains a formidable challenge. In this work, inspired by the energy dissipation mechanism and the recoverability of sacrificial bonds, the authors describe a dual-dynamic network design of a high-performance elastomer in which weaker multiple hydrogen bonds and stronger Zn–triazole coordination have been engineered into an unvulcanized cis-1,4-polyisoprene (IR) matrix. Accordingly, the elastomer obtains high tensile strength (21 MPa) and toughness (60 MJ m−3). The facilitated chain orientation in such a dual-dynamic network is finely substantiated by the molecular dynamics simulation results. Significantly, this dual-dynamic network design enables a fully cut elastomer to be healed at mild temperature. Under healing at 80 °C for 24 h, the healed elastomer regains excellent mechanical properties (tensile strength of 15.5 MPa and fracture energy of 42.8 MJ m−3). We envision that this design concept can not only develop a new network construction method in rubbers instead of vulcanization, but also provide inspiration for preparing advanced elastomers with the combination of excellent mechanical performance and high self-healing capability.


 Please wait while we load your content...
Please wait while we load your content...