Photoelectrochemistry of colloidal Cu2O nanocrystal layers: the role of interfacial chemistry†
Abstract
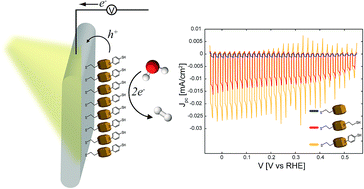
Colloidal Cu2O nanocrystal layers on Au substrates are studied as photocathodes in the context of solar electrochemical water-splitting applications. The photoelectrochemical response of the nanocrystal layers in aqueous solutions under simulated solar light conditions depends strongly on the interfacial chemistry and its impact on the transport of the charge carriers across the Au/nanocrystals/liquid interfaces. The Cu2O nanocrystals are originally stabilized with octadecylamine ligands. While octadecylamine is an efficient capping ligand for the colloidal synthesis of highly uniform nanocrystals, its low conductivity impedes the charge transport across the Au/nanocrystals/liquid interfaces. The photoresponse of the nanocrystals can be enhanced by the replacement of the octadecylamine ligands with more conductive and hydrophilic molecules, such as 1,2-ethanedithiol and benzene-1,4-dithiol. The conductivity and hydrophilicity of the ligands were investigated and found to be important for the photo-induced charge separation and transport across the Au/nanocrystals/liquid interfaces and transfer to the liquid. Furthermore, the interfacial energetics of the Au/nanocrystals/liquid junction and the resulting photoresponse of the Cu2O nanocrystal photocathode can be optimized by rational design of the exchanging ligands with desired functionalities and dipoles at the specific interfaces. A comparison of the photoresponse of Cu2O nanocrystal layers to that of electrodeposited Cu2O layers shows that the former is, yet, lower, due to the apparent low conductivity of the ligands. However, the nanocrystal organic ligands impart high hydrophobicity, which prevents the contact of the aqueous solution with the nanocrystals and improves their stability against photocorrosion and reduction to Cu0, as confirmed by X-ray diffraction measurements.


 Please wait while we load your content...
Please wait while we load your content...