Li-ion transport in a representative ceramic–polymer–plasticizer composite electrolyte: Li7La3Zr2O12–polyethylene oxide–tetraethylene glycol dimethyl ether†
Abstract
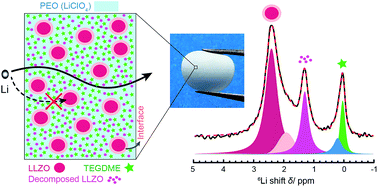
Ceramic–polymer hybrids carry the promise of forming composite electrolytes with high ionic conductivity, good stability and compatibility with electrodes, and excellent mechanical properties that cannot be achieved with conventional ceramic or polymer ion conductors. Small molecule additives often further enhance ion conduction. This work employs a representative composite comprising a ceramic Li-ion conductor, garnet-type Li7La3Zr2O12 (LLZO), a polymer Li-ion conductor, polyethylene oxide (PEO), and an additive, tetraethylene glycol dimethyl ether (TEGDME). All the Li sites in bulk LLZO, PEO, and TEGDME, and at the interfaces of PEO/LLZO, PEO/TEGDME, and LLZO/TEGDME, have been clearly identified with high-resolution Li NMR. It is determined using the 6Li → 7Li isotope replacement strategy that Li-ion transport in this composite occurs mainly via TEGDME-associated phases. Changes in the bulk and interfacial structures of the composite will likely alter Li-ion pathways and thus lead to variation in ionic conductivity. As an example, the composite structure evolves over time, which results in a decrease in active Li sites and degradation of ionic conductivity.


 Please wait while we load your content...
Please wait while we load your content...