Thermodynamics of paired charge-compensating doped ceria with superior redox performance for solar thermochemical splitting of H2O and CO2†
Abstract
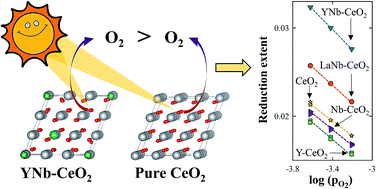
Paired charge-compensating doped ceria (PCCD) using trivalent and pentavalent cations are evaluated as redox materials for the thermochemical splitting of H2O and CO2. The oxygen nonstoichiometries of PCCD materials with formulas of Ce0.9A0.05Nb0.05O2 (A = Y, La, Sc) and CexLa(1−x)/2Nb(1−x)/2O2 (x = 0.75, 0.95) were measured in a thermogravimetric analyzer over a range of temperatures (T = 1173–1773 K) and oxygen partial pressures (pO2 = 10−15–10−1 atm). Undoped and single element doped ceria (Ce0.9B0.1O2 where B = Y, La, Nb, Hf) served as a reference. At any given set of T and pO2, the relative reduction extent follows Ce0.9Hf0.1O2 > Ce0.9Sc0.05Nb0.05O2 > Ce0.9Y0.05Nb0.05O2 > CexLa(1−x)/2Nb(1−x)/2O2 > CeO2 > solely trivalent or pentavalent doped ceria. The partial molar reduction enthalpies were determined using Van't Hoff analysis coupled to defect modeling and range from 360 to 410 kJ mol−1. A system efficiency model predicts that these PCCD materials have the potential of achieving high solar-to-fuel energy conversion efficiencies because of their balanced reduction and oxidation properties. Ce0.9Y0.05Nb0.05O2 in particular can outperform undoped ceria and reach efficiency values of 31% and 28% for H2 and CO production, respectively.


 Please wait while we load your content...
Please wait while we load your content...