High-rate capability of Na2FePO4F nanoparticles by enhancing surface carbon functionality for Na-ion batteries†
Abstract
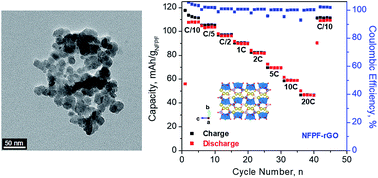
Metal phosphate compounds are considered promising candidates as positive electrode materials for Na-ion batteries because they offer higher cation-insertion potentials than analogous metal oxides. One such example is sodium iron fluorophosphate (Na2FePO4F), a compound that is typically synthesized by high-temperature solid-state routes. In this study, we prepare phase-pure Na2FePO4F using the polyol route, a low-temperature process that allows for the synthesis of nanoparticles (15–25 nm), a form that enhances Na-ion insertion kinetics and cycling stability. We then apply two methods to enhance the electronic conductivity of Na2FePO4F: (i) converting residual organic byproducts of the polyol synthesis to conductive carbon coatings; and (ii) preparing a nanocomposite with reduced graphene oxide. The resulting electrode materials are characterized in nonaqueous Na-ion electrolytes, assessing such metrics as specific capacity, rate capability, and cycling stability. A thorough electrochemical kinetics analysis is performed to deconvolve surface-vs.-bulk Na-ion insertion as a function of composite structure. Specific capacities between 60–110 mA h g−1 were achieved in galvanostatic charge–discharge tests when cycling in the range from 10C to C/10, respectively.


 Please wait while we load your content...
Please wait while we load your content...