Solvent-free synthesis of an ionic liquid integrated ether-abundant polymer as a solid electrolyte for flexible electric double-layer capacitors†
Abstract
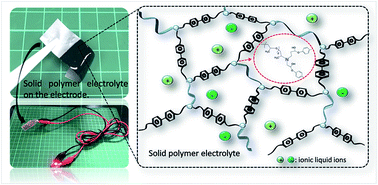
This study develops a solvent-free synthesis technique for producing a poly(ethylene oxide)-co-poly(propylene oxide) copolymer cross-linked by a bisphenol-A diglycidyl ether in an ionic liquid (IL), 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (i.e., EMImTFSI). The ether-abundant feature renders the polymer precursors soluble in the IL and enables the integration of the resulting polymer and IL involved in the synthesis into a solid polymer electrolyte (SPE). The SPE exhibits excellent mechanical and thermal stabilities and presents an electrochemical performance similar to liquid-state EMImTFSI (e.g., with an ionic conductivity of 1.38 × 10−3 S cm−1 at 20 °C). When incorporated into a flexible electric double-layer capacitor (EDLC), the SPE is directly synthesized on the carbon electrodes to enable full penetration of the SPE into the carbon micropores. The abundant ether functionalities facilitate the dissociation of the [EMIm]+[TFSI]− pair to improve the ultimate capacitance of the EDLC, despite the polymer network slightly impeding the ion transport across the carbon electrode. The high mechanical strength of the SPE is demonstrated by the negligible difference in the charge-storage performance when the flexible EDLC is bent in a range of 0–90°. This EDLC has superior specific energy and power (41.2 W h kg−1 and 12.3 kW kg−1, respectively) and excellent stability when operated within 0–3 V. The distinct merits of this synthesis technique are its environmentally friendly features and efficient integration of the SPE and electrodes, thus ensuring that this technique is readily applicable at industrial levels.


 Please wait while we load your content...
Please wait while we load your content...