Removing structural water from sodium titanate anodes towards barrier-free ion diffusion for sodium ion batteries†
Abstract
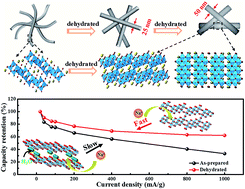
Nanostructures have been proved to be a promising strategy to realize fast Na-ion diffusion and high electrochemical activity for sodium titanate anodes. However, most researchers pay their attention to the controllable synthesis of nano-morphology for sodium titanate; there are few reports on the existence of water molecules and their influence on the structure and electrochemical performance of these solution-synthesized sodium titanate electrodes. Herein, urchin-like layered sodium titanate ultrafine nanowires are synthesized and the existence of structural water in them is confirmed. Their structural evolution upon the removal of water is investigated by SEM, XRD, TEM, IR and Raman measurements. The departure of interlayer water not only leads to a morphology change from ultrafine nanowires to highly crystalline nanorods but also induces a structure transformation from layered NaTi3O6(OH)·2H2O into tunnel structured Na2Ti6O13. The electrochemical analysis confirms that the dehydrated sample exhibits a better rate capability and enhanced cycling performance, suggesting that the existence of interlayer water in sodium titanate is unfavourable for the sodium ion diffusion in TiO6 octahedral layers. Our findings give a new understanding of the design and synthesis of the sodium titanate nanostructure with high rate capability.


 Please wait while we load your content...
Please wait while we load your content...