Evolution of hydrogen by few-layered black phosphorus under visible illumination†
Abstract
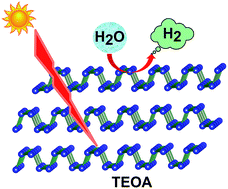
Recently, a new class of two-dimensional black phosphorus (BP) with a visible direct band gap is predicted as a potential candidate for photo-catalysis applications. Here, we present the first experimental evidence of hydrogen (H2) evolution from aqueous solution by using BP (nanosheets and nanoparticles) under visible light illumination. Our experimental results describe that liquid phase exfoliated BP nanosheets and BP nanoparticles exhibit suitable energy level alignments for electron transfer and further proton reduction reactions in the solution under visible light illumination. Density functional theory (DFT) calculations predict that the H2 evolution activity of bilayer BP is independent of edge or center positions, which is unique in BP as compared to those of other 2D materials.


 Please wait while we load your content...
Please wait while we load your content...