Catalytic action of Y2O3@graphene nanocomposites on the hydrogen-storage properties of Mg–Al alloys
Abstract
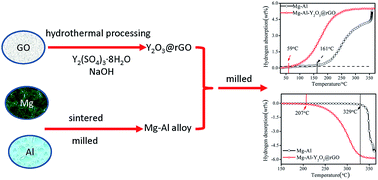
An Y2O3@rGO nanocomposite was synthesized via an impregnation method and the catalytic effect of the nanocomposite on the hydrogen-storage properties of a Mg–Al alloy was investigated. The pressure composition isotherm measurement results revealed that the Mg–Al–Y2O3@rGO composite underwent a reversible hydrogenation/dehydrogenation process at 250 °C. Furthermore, the onset temperatures of hydrogenation and dehydrogenation were significantly (i.e., 102 °C and 122 °C, respectively) lower than the respective values corresponding to the Mg–Al alloy. The Y2O3@rGO nanocomposite enhanced the hydriding kinetic properties of the alloy. The hydrogenation kinetic parameter of the Mg–Al alloy increased from 0.008 to 0.195 at 300 °C with 5 wt% of the Y2O3@rGO composite. The values of 162.6 kJ mol−1 H2 and 145.9 kJ mol−1 H2 were obtained for the dehydrogenation energy barrier (evaluated by means of a Kissinger plot) of the Mg–Al alloy and the Mg–Al–Y2O3@rGO hydride, respectively. The reaction enthalpy of hydrogenation/dehydrogenation (determined from a van't Hoff plot) of the alloy decreased with the addition of the Y2O3@rGO nanocomposite. For example, the values of 70.7 kJ mol−1 H2 and 54.3 kJ mol−1 H2 were obtained for the reaction enthalpy of hydrogenation associated with the Mg–Al alloy and the Mg–Al–Y2O3@rGO composite, respectively. Therefore, the addition of the Y2O3@rGO nanocomposite is conducive for improving the thermodynamic and kinetic properties of hydrogenation/dehydrogenation of the Mg–Al alloy.


 Please wait while we load your content...
Please wait while we load your content...