Facet-dependent photocatalytic properties of Cu2O crystals probed by using electron, hole and radical scavengers†
Abstract
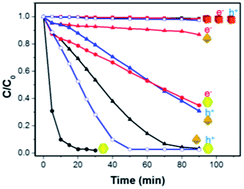
To understand the photocatalytic inactivity of Cu2O nanocubes and the superior photocatalytic activity of Cu2O rhombic dodecahedra compared to that of Cu2O octahedra, electron and hole scavengers, as well as hydroxyl and superoxide anion radical scavengers, were introduced in the photodegradation of methyl orange using these Cu2O crystals as the photocatalysts. Scavenger results suggest that photogenerated electrons and holes experience a large barrier at the {100} faces of cubes preventing charge migration to the particle surfaces, leading to the photocatalytic inactivity of Cu2O cubes. For Cu2O rhombic dodecahedra, both photogenerated electrons and holes are efficiently utilized to produce radicals, giving them excellent photocatalytic activity. In contrast, the photocatalytic activity of Cu2O octahedra mostly comes from photoexcited electrons migrating to the particle surfaces to yield superoxide anion radicals and hydroxyl radicals, while holes are largely unavailable for the photo-oxidation reaction. Ultraviolet photoelectron spectroscopy (UPS) and reflectance spectra were used to construct conventional band diagrams for these particle shapes, but such band diagrams cannot account for the large face-specific photocatalytic properties of Cu2O nanocrystals. A modified band diagram presenting different degrees of band bending at various crystal surfaces is more useful to explain the observed facet-dependent phenomena.


 Please wait while we load your content...
Please wait while we load your content...