Drastically enhanced hydrogen evolution activity by 2D to 3D structural transition in anion-engineered molybdenum disulfide thin films for efficient Si-based water splitting photocathodes†
Abstract
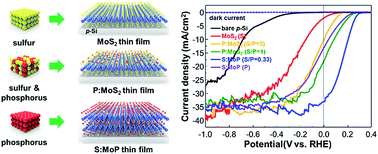
We synthesized transferrable and transparent anion-engineered molybdenum disulfide thin-film catalysts through a simple thermolysis method by using [(NH4)2MoS4] solution and powder precursors with different sulphur/phosphorus weight ratios. The synthesized sulphur-doped molybdenum phosphide (S:MoP) thin film changed from a two-dimensional van der Waals structure to a three-dimensional hexagonal structure by introduction of phosphorus atoms in the MoS2 thin film. The S:MoP thin film catalyst, which is composed of cheap and earth abundant elements, could provide the lowest onset potential and the highest photocurrent density for planar p-type Si photocathodes. The density functional theory calculations indicate that the surface of S:MoP thin films absorb hydrogen better than that of MoS2 thin films. The structurally engineered thin film catalyst facilitates the easy transfer of photogenerated electrons from the p-Si light absorber to the electrolyte. Anion-engineering of the MoS2 thin film catalyst would be an efficient way to enhance the catalytic activity for photoelectrochemical water splitting.


 Please wait while we load your content...
Please wait while we load your content...