Electrocatalytic water oxidation at low energy cost by a highly active and robust calcium–manganese oxide thin film sintered on an FTO electrode with ethyl methyl imidazolium triflate ionic liquid†
Abstract
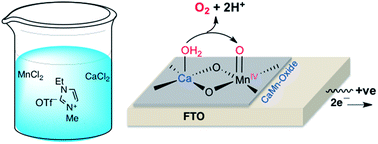
We report a simple and novel method involving an ethyl methyl imidazolium triflate ionic liquid (EMI OTf) to prepare highly mechanically stable crystalline α-Mn2O3 and calcium-incorporated manganese oxide (CaMn-oxide) thin film water oxidation electrocatalysts (WOCs) on a fluorine-doped tin oxide (FTO) electrode (FTO/EMI α-Mn2O3 and FTO/EMI CaMn-oxide). Electrochemical measurements showed that the incorporation of calcium into manganese oxide promotes water oxidation at a very low energy cost, similar to that observed in natural photosystem II (PSII). The low energy cost is due to the involvement of the MnIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species in the catalysis as compared to the MnV
O species in the catalysis as compared to the MnV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species that has been previously detected in calcium-free α-Mn2O3. Moreover, the FTO/EMI CaMn-oxide electro-catalyst showed unprecedentedly high activity (TOF ≈ 29.8 s−1 at η = 0.57 V) and robustness (tested for 40 h) for water oxidation in a neutral potassium phosphate (KPi) buffer solution (0.1 M, pH 7.0). The mechanism of water oxidation by FTO/EMI CaMn-oxide was discussed and applied for water oxidation by the μ-oxido-CaMn4O5 cluster of photosystem II (PS II).
O species that has been previously detected in calcium-free α-Mn2O3. Moreover, the FTO/EMI CaMn-oxide electro-catalyst showed unprecedentedly high activity (TOF ≈ 29.8 s−1 at η = 0.57 V) and robustness (tested for 40 h) for water oxidation in a neutral potassium phosphate (KPi) buffer solution (0.1 M, pH 7.0). The mechanism of water oxidation by FTO/EMI CaMn-oxide was discussed and applied for water oxidation by the μ-oxido-CaMn4O5 cluster of photosystem II (PS II).


 Please wait while we load your content...
Please wait while we load your content...