K-doped Sr2Fe1.5Mo0.5O6−δ predicted as a bifunctional catalyst for air electrodes in proton-conducting solid oxide electrochemical cells†
Abstract
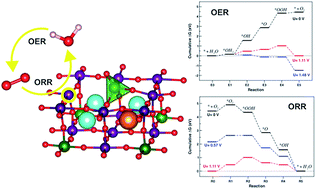
Perovskite oxides are promising electrodes for oxygen evolution and reduction reactions (OER/ORR), but it is difficult for a single material to catalyze both reactions efficiently. We investigated using first-principles calculations the K-doped Sr2Fe1.5Mo0.5O6−δ mixed proton–electron conducting oxide. We found that aliovalent doping tunes the electronic features to be optimal for the ORR and lattice expansion-driven surface reconstruction stabilizes the key *OOH intermediate for the OER. The resulting ORR/OER overpotentials are very low (∼0.5 V), suggesting the application of this material as an air electrode in reversible solid oxide electrochemical cells.


 Please wait while we load your content...
Please wait while we load your content...