Water splitting in near-neutral media: using an Mn–Co-based nanowire array as a complementary electrocatalyst†
Abstract
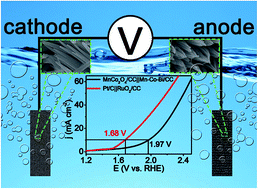
Developing earth-abundant electrocatalysts for efficient full water splitting under benign conditions is highly desired but still a significant challenge. In this communication, we report the development of a Mn–Co-based nanowire array as a complementary catalyst for efficient water splitting in near-neutral media. The Mn–Co–borate nanowire array on carbon cloth (Mn–Co–Bi/CC) was prepared via oxidative polarization of a MnCo2O4 nanowire array (MnCo2O4) in potassium borate (K-Bi), behaving as an efficient electrocatalyst for water oxidation with an overpotential of 366 mV to drive a catalytic current density of 10 mA cm−2 in 0.5 M K-Bi (pH: 9.2). MnCo2O4/CC exhibits outstanding hydrogen evolution activity requiring an overpotential of 293 mV at 10 mA cm−2 in 0.5 M K-Bi. The two-electrode electrolyser using Mn–Co–Bi/CC as the anode and MnCo2O4/CC as the cathode drives 10 mA cm−2 at a cell voltage of 1.97 V with strong long-term electrochemical durability and 100% faradaic efficiency.


 Please wait while we load your content...
Please wait while we load your content...