Y-Doped Na3V2(PO4)2F3 compounds for sodium ion battery cathodes: electrochemical performance and analysis of kinetic properties
Abstract
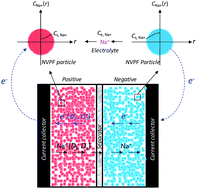
To improve the intrinsic electronic conductivity and Na ion mobility of Na3V2(PO4)2F3 (NVPF), Y(yttrium) atoms are introduced into the NVPF/C complex as a partial substitute for V(vanadium) through a sol–gel method. The effects of Y substitution on the crystal structure, morphology, electrochemical performance and kinetic properties of NVPF were investigated. Based on the battery performance comparison of the Na3V2−xYx(PO4)3/C (x = 0, 0.05, 0.1 and 0.2) samples, Na3V1.9Y0.1(PO4)3/C showed the best electrochemical performance and cycling stability. At a low rate of 0.5C, the 5 mol% Y-doped sample delivered a discharge capacity of 121.3 mA h g−1, which was very close to the theoretical specific capacity. And even at a high rate of 50C, the discharge capacity achieved was higher than 80 mA h g−1. After 200 cycles, the capacity retention of Na3V1.9Y0.1(PO4)3/C could still remain as high as 93.46% at 1C. From the morphology determination and analysis of kinetic properties, it was confirmed that the excellent electrochemical performance of Na3V1.9Y0.1(PO4)3/C was mainly due to the enhanced intrinsic electronic conductivity and Na ion mobility caused by introducing a moderate amount of Y to replace the V sites in the NVPF crystal structure. In order to get a better understanding of the relationship between the kinetic properties and the electrochemical performance in a sodium ion battery, a mass and electron transfer process model has been proposed for the first time in the present research.


 Please wait while we load your content...
Please wait while we load your content...