In situ surface engineering of nickel inverse opal for enhanced overall electrocatalytic water splitting†
Abstract
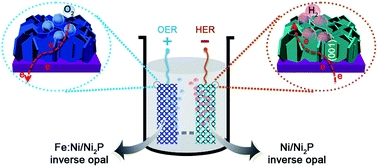
High-efficiency non-precious catalysts are important for hydrogen and oxygen evolution reactions (HER and OER). Practical water splitting needs not only intrinsically active catalyst materials but also the maximization of their electrocatalytic capability in a real electrolyzer. Here, we report for the first time a Ni/Ni2P inverse opal architecture fabricated by surface engineering. The superior HER properties are enabled by maximum active crystallographic plane exposure and vertical alignment of Ni2P nanosheets on nickel inverse opal. It requires an overpotential of only 73 mV to drive a HER current density of −20 mA cm−2. After doping with Fe, the resulting Fe:Ni/Ni2P inverse electrode shows excellent OER performance with a very low overpotential (285 mV) at a current density of 20 mA cm−2. An alkaline electrolyzer using the two 3D structured electrodes could split water at 20 mA cm−2 with a low voltage of ∼1.52 V for 100 h. The catalytic activity is even superior to that of the noble metal catalyst couple (IrO2–Pt/C). This work provides a surface engineered opal structure to maximize the electrocatalyst properties in the systems with coupled electron transfer and mass transport.


 Please wait while we load your content...
Please wait while we load your content...