Engineering hollow polyhedrons structured from carbon-coated CoSe2 nanospheres bridged by CNTs with boosted sodium storage performance†
Abstract
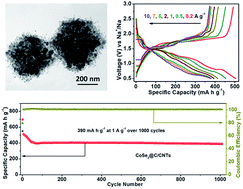
Nanostructured CoSe2 anode materials hold great promise for sodium ion batteries (SIBs), drawing much recent research attention. However, high-performance CoSe2 based anodes are still challenging to obtain. Herein, using zeolitic imidazolate framework-67 (ZIF-67) particles as the starting material, nondestructive hollow polyhedral hybrids have been synthesized successfully, which are structured from CNT-bridged carbon-coated CoSe2 nanospheres (CoSe2@C/CNTs). During the synthesis, the controlled in situ growth of CNTs introduces additional mesopores and open channels to the hybrids, and avoids serious agglomeration of the CoSe2 nanospheres. When employed as anode materials for SIBs with ether-based electrolyte, the CoSe2@C/CNTs show overwhelming merits over graphitic carbon-coated CoSe2 nanosphere polyhedral hybrids (CoSe2@GC) and bare CoSe2 particles. Specifically, the CoSe2@C/CNTs anode displays a high reversible capacity (∼470 mA h g−1 at 0.2 A g−1), a good rate capability of ∼373 mA h g−1 even at 10 A g−1, and an excellent cycling stability of over 1000 cycles with a capacity retention of ∼100% calculated from the 70th cycle. In addition, the electrochemical reaction dynamics analysis indicates a considerable capacitive contribution during the discharge–charge cycles, which is beneficial to enhance the rate capability and cyclability of the CoSe2@C/CNTs anode. Such results could be ascribed to the stable ether-based electrolyte-active material intermediates, improved electrolyte-active material contact, and shortened charge transfer paths afforded by the unique hybrid nanostructure.


 Please wait while we load your content...
Please wait while we load your content...