Lithium migration between blended cathodes of a lithium-ion battery†
Abstract
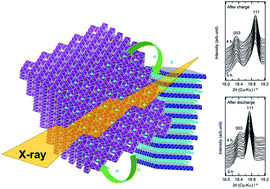
Lithium migration in electrodes of a lithium-ion battery (LIB) is a necessary electrochemical process to store energy in the battery. An understanding of the mechanism of lithium migration can lead to an improvement in LIBs and the development of next-generation rechargeable batteries. In general, two cathode materials are frequently used in the cathode of an LIB. We found a unique behavior for lithium migration in a blended 10 cathode consisting of LiAl0.1Mn1.9O4 (LMO) and LiNi1/3Mn1/3Co1/3O2 (NMC) during and after charge and discharge processes via in situ X-ray diffraction. First, the relationships among the capacities, open-circuit voltages (Voc), and lattice parameters were investigated for the cathodes composed of either LMO or NMC. Subsequently, their lattice parameters in the blended cathodes were determined to evaluate the capacity and Voc of each active material. The capacities of LMO and NMC in the blended cathodes can be deduced from the lattice parameters. Furthermore, the sums of their capacities coincided with the cell capacities. In addition, changes in the lattice parameters of NMC were observed during voltage relaxation after the charge and discharge processes, which revealed that lithium migration occurred between LMO and NMC. These results demonstrate that charge–discharge processes locally occur at the interface between the materials in the blended cathode after the charge and discharge processes are stopped.


 Please wait while we load your content...
Please wait while we load your content...