Metal–organic framework – derived Co9S8@CoS@CoO@C nanoparticles as efficient electro- and photo-catalysts for the oxygen evolution reaction†
Abstract
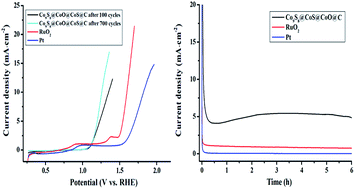
Through an annealing method, Co9S8@CoS@CoO@C nanoparticles (NPs) of 5–20 nm size were synthesized using a metal–organic framework (MOF) as the precursor. The Co9S8@CoS@CoO@C NPs exhibited excellent electrocatalytic activity for the oxygen evolution reaction (OER) with a lower overpotential than RuO2 (0.37 V) and Pt NPs (0.60 V). The better electrocatalytic activity of the hybrid material is associated with its heterojunction structure, in which the strong electron coupling interactions between Co9S8, CoS, CoO and C give rise to a superior synergistic effect. The interconnected amorphous carbon not only anchored the active Co compounds to avoid aggregation, but also afforded conducting channels for electron transfer. Furthermore, the cobalt compounds were the active species in the sample during the OER process; they were converted into CoOOH and Co(OH)2 in the KOH electrolyte, and the S elements in the sample entered the solution. The Co9S8@CoS@CoO@C NPs can exhibit the best OER activity with minimum overpotential (0.028 V) to afford a stable OER current after activation in a KOH aqueous solution. The sample also showed obvious photoresponse to visible light irradiation with enhanced OER current and lowered onset potential, which was probably associated with the semiconducting characteristics of the spin-polarized CoO and the quantum confinement effect of the nanoparticles.


 Please wait while we load your content...
Please wait while we load your content...