Ab initio investigation of Jahn–Teller-distortion-tuned Li-ion migration in λ-MnO2
Abstract
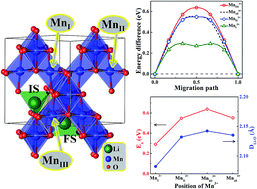
The migration of Li ions in electrode materials is the main limiting factor to determine the rate capability of Li-ion batteries. In this work, the influences of Jahn–Teller (JT) distortion on Li migration in full delithiated LiMn2O4 (λ-MnO2) were systematically studied by a first-principles computational approach. Our results unravel the direction of JT distortion strongly affecting the activation barrier of Li-ion migration in λ-MnO2. In particular, Li-ion migration has the lowest activation barrier when the two elongated Mn–O bonds of Mn3+ ion are quasi-collinear with the linked Li–O bonds in the transition state, in contrast with the highest barrier when the elongated Mn–O bonds are approximately perpendicular to the linked Li–O bonds. In addition, lattice-strain-induced variation of the Li-migration barrier in λ-MnO2 exhibits either upward or downward trends, depending on the detailed coupling with JT distortion. Further analysis showed that the difference in activation barriers can be explained by the different Li–O distances in terms of the Coulomb interaction energies, which is induced by the different position and direction of JT distortion. Finally, the Li-ion migration in the whole λ-MnO2 system is also discussed by considering the influences of JT polarons.


 Please wait while we load your content...
Please wait while we load your content...