The structure and unconventional dihydrogen bonding of a pressure-stabilized hydrogen-rich (NH3BH3)(H2)x (x = 1.5) compound†
Abstract
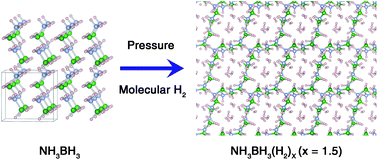
Combining X-ray diffraction, Raman spectroscopy, and ab initio simulations we characterize an extremely hydrogen-rich phase with the chemical formula (NH3BH3)(H2)x (x = 1.5). This phase was formed by compressing ammonia borane (AB, NH3BH3) in an environment with an excess of molecular hydrogen (H2). This compound can store a total of 26.8 wt% hydrogen, both as molecular hydrogen and chemically bonded hydrogen in AB, making it one of the most hydrogen-rich solids currently known. The new compound possesses a layered AB structure where additional H2 molecules reside in channels created through the weaving of AB layers. The unconventional dihydrogen bonding network of the new compound is significantly modified from its parent AB phase and contains H⋯H contacts between adjacent AB molecules and between AB and H2 molecules. H–H can be either a proton donor or a proton acceptor that forms new types of dihydrogen bonding with the host AB molecules, which are depicted as H–H⋯H–B or H–H⋯H–N, respectively. This study not only demonstrates the strategy and the promise of using pressure for new material synthesis, but also unleashes the power of combining experiments and ab initio calculations for elucidating novel structures and unusual bonding configurations in dense low-Z materials.


 Please wait while we load your content...
Please wait while we load your content...