5-(Dinitromethyl)-3-(trinitromethyl)-1,2,4-triazole and its derivatives: a new application of oxidative nitration towards gem-trinitro-based energetic materials†
Abstract
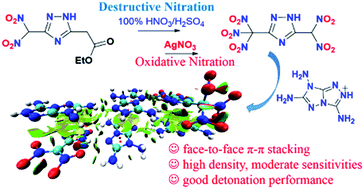
An excellent method for the preparation of gem-trinitro compounds is destructive nitration based on an active methylene moiety with a neighboring carbonyl structure. Now described is an alternative approach where oxidative nitration was utilized to synthesize gem-trinitro compounds. Using this strategy, 5-(dinitromethyl)-3-(trinitromethyl)-1,2,4-triazole (6) and its ionic derivatives (7–12) were designed, prepared and thoroughly characterized. This family of new energetic materials exhibits excellent densities, good detonation performances and moderate mechanical sensitivities. Interestingly, as supported by the X-ray structure of energetic salt 8, the amine-based fused ring cation induces a face-to-face π–π arrangement thus reducing the sensitivity of the entire molecule, and advancing the further application of similar fused ring ionic stabilizers for polynitro azole anions. These results open a new chapter concerning gem-trinitro compounds in the energetic materials field.


 Please wait while we load your content...
Please wait while we load your content...