Elucidation of the reaction mechanisms of isostructural FeSn2 and CoSn2 negative electrodes for Na-ion batteries†
Abstract
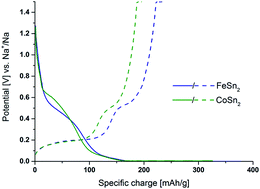
Alternatives to hard carbons need to be identified to improve the energy density of Na-ion batteries. Negative electrode materials based on intermetallics generally outperform different carbon-based electrodes. They have the advantage of a high gravimetric capacity (>500 mA h g−1) and a very high volumetric capacity, due to their density. However, these advantages are counter balanced by their lack of stability due to the large volume changes they experience along cycling. FeSn2 and CoSn2 are investigated as negative electrode materials for Na-ion batteries. The 1st cycle and the 10th cycle of the Na/MSn2 reaction are probed using operando XRD coupled to XAS to reveal the formation and ageing reaction mechanisms occurring along cycling. The role of the transition metal and the number of electrons involved in both materials reveal different reaction pathways leading to the recombination of FeSn2 at the end of the desodiation, whereas CoSn2 is unable to recombine and thus its electrochemical performance fades faster.


 Please wait while we load your content...
Please wait while we load your content...