Self-extinguishing electrolytes using fluorinated alkyl phosphates for lithium batteries†
Abstract
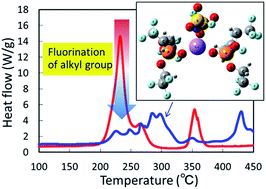
Conventional Li-ion batteries that use flammable organic electrolytes pose a severe risk to battery safety. One strategy to address this problem has been the application of self-extinguishing solvents, such as trimethyl phosphate; however, it is now apparent that these solvents result in poor Li-ion insertion and instability under overcharge. Here, we investigate some electrolytes based on fluorinated alkyl phosphates, a mixture of tris(trifluoroethyl) phosphate (TFEP) and lithium bis(fluorosulfonyl) amide (LiFSA) with various ratios [TFEP]/[LiFSA] < 8. A super-concentration with [TFEP]/[LiFSA] = 2 enabled the insertion of Li-ions into the graphite anode. The fluorination of the alkyl groups in the phosphate molecule was very effective in improving the thermal stability in the presence of charged cathode and anode active materials. In particular, the release of gases and heat that typically accompanied the reaction of the alkyl phosphate with the charged graphite anode was inhibited significantly. In addition, half cells were fabricated using the super-concentrated electrolyte with [solvent]/[salt] = 2, and the cells were demonstrated to be rechargeable at temperatures as high as 120 °C.


 Please wait while we load your content...
Please wait while we load your content...