Efficient photocatalytic reduction of dinitrogen to ammonia on bismuth monoxide quantum dots†
Abstract
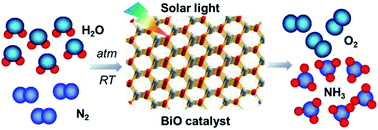
N2 reduction to ammonia by solar light represents a green and sustainable ammonia synthesis approach which helps to suppress the global warming and energy crisis. However, conventional semiconductors usually suffer from low activity or poor stability, largely suppressing the application of this technology. Here, we report that bismuth monoxide (BiO) quantum dots with an average size of 2–5 nm exhibited efficient photocatalytic activity for ammonia synthesis under simulated solar light. A highly efficient ammonia synthesis rate of 1226 μmol g−1 h−1 is achieved without the assistance of any sacrificial agent or co-catalyst, which is about 1000 times higher than that using the traditional Fe-TiO2 photocatalyst. Kinetic analysis reveals that the synergy of three low valence surface Bi(II) species markedly enhances N2 activation by electron donation, which finally resulted in the highly efficient N2 photoreduction performance. This work will shed light on designing efficient and robust N2 reduction photocatalysts.


 Please wait while we load your content...
Please wait while we load your content...