Metal ion-containing C3N3S3 coordination polymers chemisorbed to a copper surface as acid stable hydrogen evolution electrocatalysts†
Abstract
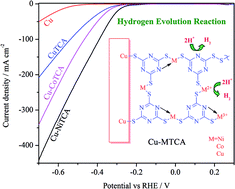
The design of stable and efficient non-noble metal–organic electrocatalytic surfaces are in focus for hydrogen evolution at low overpotentials in strong acidic conditions. We present here the preparation of a novel chemically immobilized mixed-metal ion-containing triazine thiolate (C3N3S3) polymer electrocatalyst (M–TCA) on a copper (Cu) surface. Structural investigation of the metallopolymers evidences the presence of mixed valence states (Ni2+/3+, Co2+/3+, and Cu+/2+) of metal ions. The metal ions linked to metallopolymeric networks exhibit stability and an improved hydrogen evolution activity in strong acidic electrolytes. The simultaneous proton and electron transfer at the redox active metal centers and the nitrogen rich ligand as a proton relay improve the hydrogen evolution activity. Substantial overpotential reduction and enhancement in hydrogen evolution current density were observed with the Ni ion-containing metallopolymer. This metallopolymer requires just −270 mV vs. RHE overpotential to attain 10 mA cm−2 current density in 0.5 M H2SO4 (whilst a bare Cu electrode requires −560 mV vs. RHE). Electrochemical impedance measurements reveal almost a forty-fold decrease in charge transfer resistance with Cu/M–TCA in comparison with the bare Cu electrode. The surface distributed metal ion centers and the nitrogen-rich triazine core provide active electrocatalytic sites for enhanced hydrogen evolution reaction. These chemically bound metallopolymers on the Cu surface show a remarkable durability and are firmly adhered onto the electrode surface after prolonged hydrogen evolution.


 Please wait while we load your content...
Please wait while we load your content...