Effect of fixed charge group concentration on equilibrium ion sorption in ion exchange membranes†
Abstract
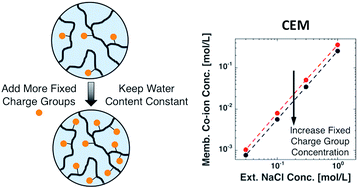
Despite their increasing importance in many energy and water purification applications, few systematic studies of ion sorption in ion exchange membranes exist where fixed charge group concentration and water content are varied independently. Such studies are critical for developing fundamental structure/property relations important for rationally tailoring such materials. Here, cation and anion exchange membranes having different fixed charge group concentrations but similar water content were synthesized to investigate the influence of fixed charge group concentration on equilibrium ion sorption in such materials. Co-ion sorption decreased with increasing membrane fixed charge group concentration, as expected, presumably due to enhanced Donnan exclusion. However, the extent to which co-ion sorption was suppressed was different for the cation and anion exchange membranes, despite similar changes in membrane fixed charge group concentration. A thermodynamic model, based on Donnan theory and Manning's counter-ion condensation theory, was used to interpret the data. The model predicted equilibrium co-ion concentrations in the anion exchange membranes with no adjustable parameters. However, good agreement between the model and experimental data for the cation exchange membranes was only obtained by treating the Manning parameter as an adjustable constant, presumably due to phase separation during polymerization, which produced inhomogeneous membranes.


 Please wait while we load your content...
Please wait while we load your content...