Iron oxide/porous carbon as a heterogeneous Fenton catalyst for fast decomposition of hydrogen peroxide and efficient removal of methylene blue†
Abstract
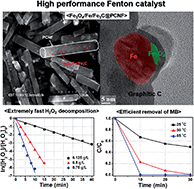
A high performance Fe3O4/Fe/Fe3C@PCNF heterogeneous Fenton catalyst was synthesized for aqueous H2O2 decomposition and was utilized to remove methylene blue in water. Surprisingly, Fe3O4/Fe/Fe3C@PCNF showed an extremely fast decomposition rate; this rate ranked as the highest value reported to date. Furthermore, it showed efficient removal of methylene blue in water which was tested at different H2O2 concentrations and solution temperatures. Based on detailed characterization, several unique features of this catalyst were presumed to be responsible for its remarkable catalytic activity for H2O2 decomposition as follows: (1) the presence of Fe0 and delocalized π-electrons of graphitic layers promotes the reduction of Fe3+ to Fe2+; (2) the catalyst particles were partially covered with a few defective graphitic layers, and H2O2 decomposition could occur through these defects; (3) the defective graphitic layers partially covered the catalyst particle to force the decomposition reaction to occur only in the vicinity of carbon and Fe3O4. Finally, this presumption was proved through experiments by changing the activation time of Fe3O4/Fe/Fe3C@PCNF. After H2O2 decomposition was completed, Fe3O4/Fe/Fe3C@PCNF could be efficiently separated by an external magnetic field because of its ferromagnetic behavior. Fe3O4/Fe/Fe3C@PCNF showed high removal activity of aqueous methylene blue tested under various conditions.

 Please wait while we load your content...
Please wait while we load your content...