Fabrication of chiroptically switchable films via co-gelation of a small chiral gelator with an achiral azobenzene-containing polymer†
Abstract
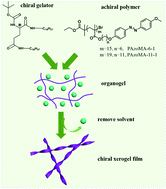
Helical polymers are widely found in nature and synthetic functional materials. Although a number of elaborate strategies have been developed to endow polymers with helicity through either covalent bonds or supramolecular techniques, it still remains a challenge to get the desired helical polymers with controlled handedness in an easy but effective manner. In this study, we report an easily accessible gelation-guided self-assembly system where the chirality of a gelator can be easily transferred to an achiral azobenzene-containing polymer during gelation. It is found that during the process of chiral induction, the induced chirality of the polymer was entirely dominated by the molecular chirality of the gelator. Experimentally, achiral azobenzene-containing polymers with different side-chain lengths were doped into a supramolecular gel system formed with amphiphilic N,N′-bis-(octadecyl)-L(D)-Boc-glutamic (LBG-18 or DBG-18 for short). CD spectra and SEM observation confirmed that the co-assembly of polymer/LBG-18 or polymer/DBG-18 in the xerogel state exhibited supramolecular chirality. More importantly, alternate UV and visible light irradiation on the xerogel film caused the induced CD signal to switch between on and off states. Thus a chiroptical switch was fabricated based on the isomerization of the azo-polymer in xerogel films.


 Please wait while we load your content...
Please wait while we load your content...