Asymmetric tin–vanadium redox electrolyte for hybrid energy storage with nanoporous carbon electrodes†
Abstract
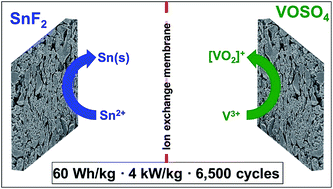
In recent decades, redox-active electrolytes have been applied in stationary energy storage systems, benefitting from Faradaic reactions of the electrolyte instead of the electrode material. One of the challenging tasks is to balance the redox activities between the negative and positive electrode. As a possible solution, a mixed electrolyte with vanadyl and tin sulfate was previously suggested; however, a low power performance is a great challenge to be overcome. Here, we found that the origin of the poor power performance in the mixture electrolyte system (vanadium complex and tin solution) is the reduction of the pore volume at the positive electrode via irreversible tin dioxide formation. To prevent the latter, we introduce a hybrid energy storage system exhibiting both battery-like and supercapacitor-like features via asymmetric redox electrolytes at the microporous activated carbon electrodes; SnF2 solution as anolyte and VOSO4 as catholyte. By employing an anion exchange membrane, the irreversible SnO2 formation at the positive electrode is effectively suppressed; thus, an asymmetric 1 M SnF2|3 M VOSO4 system provides a high maximum specific power (3.8 kW kg−1 or 1.5 kW L−1), while still exhibiting a high maximum specific energy up to 58.4 W h kg−1 (23.4 W h L−1) and a high cycling stability over 6500 cycles.


 Please wait while we load your content...
Please wait while we load your content...