Fluorogenic protein labeling using a genetically encoded unstrained alkene†
Abstract
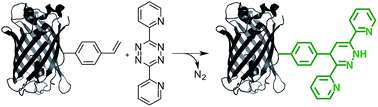
We developed a new fluorogenic bioorthogonal reaction that is based on the inverse electron-demand Diels–Alder reaction between styrene (an unstrained alkene) and a simple tetrazine. The reaction forms a new fluorophore with no literature precedent. We have identified an aminoacyl-tRNA synthetase/tRNA pair for the efficient and site-specific incorporation of a styrene-containing amino acid into proteins in response to amber nonsense codon. Fluorogenic labeling of purified proteins and intact proteins in live cells were demonstrated. The fluorogenicity of the styrene–tetrazine reaction can be potentially applied to the study of protein folding and function under physiological conditions with low background fluorescence interference.


 Please wait while we load your content...
Please wait while we load your content...