A mitochondrial-targeted prodrug for NIR imaging guided and synergetic NIR photodynamic-chemo cancer therapy†
Abstract
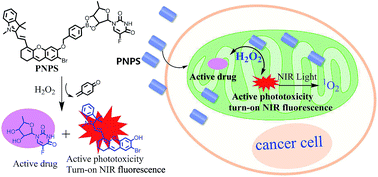
Nontoxic prodrugs, especially activated by tumor microenvironment, are urgently required for reducing the side effects of cancer therapy. And combination of chemo-photodynamic therapy prodrugs show effectively synergetic therapeutic efficiency, however, this goal has not been achieved in a single molecule. In this work, we developed a mitochondrial-targeted prodrug PNPS for near infrared (NIR) fluorescence imaging guided and synergetic chemo-photodynamic precise cancer therapy for the first time. PNPS contains a NIR photosensitizer (NPS) and an anticancer drug 5′-deoxy-5-fluorouridine (5′-DFUR). These two parts are linked and caged through a bisboronate group, displaying no fluorescence and very low cytotoxicity. In the presence of H2O2, the bisboronate group is broken, resulting in activation of NPS for NIR photodynamic therapy and activation of 5′-DFUR for chemotherapy. The activated NPS can also provide a NIR fluorescence signal for monitoring the release of activated drug. Taking advantage of the high H2O2 concentration in cancer cells, PNPS exhibits higher cytotoxicity to cancer cells than normal cells, resulting in lower side effects. In addition, based on its mitochondrial-targeted ability, PNPS exhibits enhanced chemotherapy efficiency compare to free 5′-DFUR. It also demonstrated a remarkably improved and synergistic chemo-photodynamic therapeutic effect for cancer cells. Moreover, PNPS exhibits excellent tumor microenvironment-activated performance when intravenously injected into tumor-bearing nude mice, as demonstrated by in vivo fluorescence imaging. Thus, PNPS is a promising prodrug for cancer therapy based on its tumor microenvironment-activated drug release, synergistic therapeutic effect and “turn-on” NIR imaging guide.


 Please wait while we load your content...
Please wait while we load your content...