Total syntheses of schilancidilactones A and B, schilancitrilactone A, and 20-epi-schilancitrilactone A via late-stage nickel-catalyzed cross coupling†
Abstract
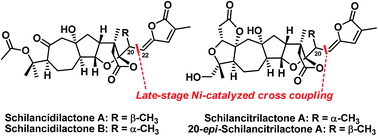
The first total syntheses of schilancidilactones A and B, schilancitrilactone A, and 20-epi-schilancitrilactone A have been accomplished using a nickel-catalyzed cross coupling of alkyl bromide with vinyl stannane as the final step. The other key steps include late-stage C(sp3)–H bromination, the oxidative cleavage of a diol to provide the requisite ketone and ester for schilancidilactones A and B, and Dieckmann-type condensation to generate the A ring of schilancitrilactone A and 20-epi-schilancitrilactone A.
- This article is part of the themed collections: Celebrating 100 Years of Chemistry at Nankai University and In celebration of Chinese New Year


 Please wait while we load your content...
Please wait while we load your content...