Unveiling the role of boroxines in metal-free carbon–carbon homologations using diazo compounds and boronic acids†
Abstract
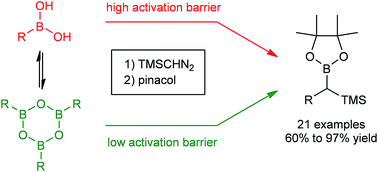
By means of computational and experimental mechanistic studies the fundamental role of boroxines in the reaction between diazo compounds and boronic acids was elucidated. Consequently, a selective metal-free carbon–carbon homologation of aryl and vinyl boroxines using TMSCHN2, giving access to TMS-pinacol boronic ester products, was developed.


 Please wait while we load your content...
Please wait while we load your content...