Solid-state molecular organometallic chemistry. Single-crystal to single-crystal reactivity and catalysis with light hydrocarbon substrates†
Abstract
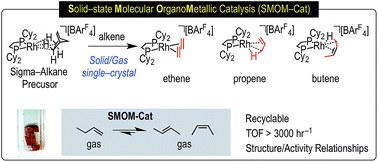
Single-crystal to single-crystal solid/gas reactivity and catalysis starting from the precursor sigma-alkane complex [Rh(Cy2PCH2CH2PCy2)(η2η2-NBA)][BArF4] (NBA = norbornane; ArF = 3,5-(CF3)2C6H3) is reported. By adding ethene, propene and 1-butene to this precursor in solid/gas reactions the resulting alkene complexes [Rh(Cy2PCH2CH2PCy2)(alkene)x][BArF4] are formed. The ethene (x = 2) complex, [Rh(Cy2PCH2CH2PCy2)(ethene)2][BArF4]-Oct, has been characterized in the solid-state (single-crystal X-ray diffraction) and by solution and solid-state NMR spectroscopy. Rapid, low temperature recrystallization using solution methods results in a different crystalline modification, [Rh(Cy2PCH2CH2PCy2)(ethene)2][BArF4]-Hex, that has a hexagonal microporous structure (P6322). The propene complex (x = 1) [Rh(Cy2PCH2CH2PCy2)(propene)][BArF4] is characterized as having a π-bound alkene with a supporting γ-agostic Rh⋯H3C interaction at low temperature by single-crystal X-ray diffraction, variable temperature solution and solid-state NMR spectroscopy, as well as periodic density functional theory (DFT) calculations. A fluxional process occurs in both the solid-state and solution that is proposed to proceed via a tautomeric allyl-hydride. Gas/solid catalytic isomerization of d3-propene, H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCD3, using [Rh(Cy2PCH2CH2PCy2)(η2η2-NBA)][BArF4] scrambles the D-label into all possible positions of the propene, as shown by isotopic perturbation of equilibrium measurements for the agostic interaction. Periodic DFT calculations show a low barrier to H/D exchange (10.9 kcal mol−1, PBE-D3 level), and GIPAW chemical shift calculations guide the assignment of the experimental data. When synthesized using solution routes a bis-propene complex, [Rh(Cy2PCH2CH2PCy2)(propene)2][BArF4], is formed. [Rh(Cy2PCH2CH2PCy2)(butene)][BArF4] (x = 1) is characterized as having 2-butene bound as the cis-isomer and a single Rh⋯H3C agostic interaction. In the solid-state two low-energy fluxional processes are proposed. The first is a simple libration of the 2-butene that exchanges the agostic interaction, and the second is a butene isomerization process that proceeds via an allyl-hydride intermediate with a low computed barrier of 14.5 kcal mol−1. [Rh(Cy2PCH2CH2PCy2)(η2η2-NBA)][BArF4] and the polymorphs of [Rh(Cy2PCH2CH2PCy2)(ethene)2][BArF4] are shown to be effective in solid-state molecular organometallic catalysis (SMOM-Cat) for the isomerization of 1-butene to a mixture of cis- and trans-2-butene at 298 K and 1 atm, and studies suggest that catalysis is likely dominated by surface-active species. [Rh(Cy2PCH2CH2PCy2)(η2η2-NBA)][BArF4] is also shown to catalyze the transfer dehydrogenation of butane to 2-butene at 298 K using ethene as the sacrificial acceptor.
CHCD3, using [Rh(Cy2PCH2CH2PCy2)(η2η2-NBA)][BArF4] scrambles the D-label into all possible positions of the propene, as shown by isotopic perturbation of equilibrium measurements for the agostic interaction. Periodic DFT calculations show a low barrier to H/D exchange (10.9 kcal mol−1, PBE-D3 level), and GIPAW chemical shift calculations guide the assignment of the experimental data. When synthesized using solution routes a bis-propene complex, [Rh(Cy2PCH2CH2PCy2)(propene)2][BArF4], is formed. [Rh(Cy2PCH2CH2PCy2)(butene)][BArF4] (x = 1) is characterized as having 2-butene bound as the cis-isomer and a single Rh⋯H3C agostic interaction. In the solid-state two low-energy fluxional processes are proposed. The first is a simple libration of the 2-butene that exchanges the agostic interaction, and the second is a butene isomerization process that proceeds via an allyl-hydride intermediate with a low computed barrier of 14.5 kcal mol−1. [Rh(Cy2PCH2CH2PCy2)(η2η2-NBA)][BArF4] and the polymorphs of [Rh(Cy2PCH2CH2PCy2)(ethene)2][BArF4] are shown to be effective in solid-state molecular organometallic catalysis (SMOM-Cat) for the isomerization of 1-butene to a mixture of cis- and trans-2-butene at 298 K and 1 atm, and studies suggest that catalysis is likely dominated by surface-active species. [Rh(Cy2PCH2CH2PCy2)(η2η2-NBA)][BArF4] is also shown to catalyze the transfer dehydrogenation of butane to 2-butene at 298 K using ethene as the sacrificial acceptor.
- This article is part of the themed collection: Celebrating our 2019 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...