N,N-Dimethylation of nitrobenzenes with CO2 and water by electrocatalysis†
Abstract
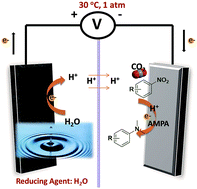
We have proposed a strategy for the synthesis of N,N-dimethylanilines from nitrobenzene and its derivatives, CO2, and water via an electrochemical reaction under ambient conditions. H+ generated from H2O was used as the hydrogen source. Pd/Co–N/carbon, in which the Pd nanoparticles were supported on Co–N/carbon, was designed and used as the electrocatalyst. It was found that the electrocatalyst was very efficient for the reaction in MeCN solution with 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([Bmim]Tf2N) as the supporting electrolyte and 1-amino-methylphosphonic acid (AMPA) as the thermal co-catalyst. A series of control experiments showed that Pd/Co–N/carbon and AMPA cooperated very well in accelerating the reaction. This synthetic route has some obvious advantages, such as using CO2 and water as the reactants, ambient reaction conditions, and high yields of the desired products. This opens up a way to synthesize chemicals by the combination of an electrocatalyst and a thermal catalyst with organic compounds, CO2, and water as the reactants.


 Please wait while we load your content...
Please wait while we load your content...