A bottom up approach towards artificial oxygenases by combining iron coordination complexes and peptides†
Abstract
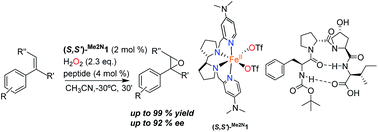
Supramolecular systems resulting from the combination of peptides and a chiral iron coordination complex catalyze asymmetric epoxidation with aqueous hydrogen peroxide, providing good to excellent yields and high enantioselectivities in short reaction times. The peptide is shown to play a dual role; the terminal carboxylic acid assists the iron center in the efficient H2O2 activation step, while its β-turn structure is crucial to induce high enantioselectivity in the oxygen delivering step. The high level of stereoselection (84–92% ee) obtained by these supramolecular catalysts in the epoxidation of 1,1′-alkyl ortho-substituted styrenes, a notoriously challenging class of substrates for asymmetric catalysis, is not attainable with any other epoxidation methodology described so far. The current work, combining an iron center ligated to N and O based ligands, and a peptide scaffold that shapes the second coordination sphere, may be seen as a bottom up approach towards the design of artificial oxygenases.


 Please wait while we load your content...
Please wait while we load your content...