Zwitterionic amidinates as effective ligands for platinum nanoparticle hydrogenation catalysts†
Abstract
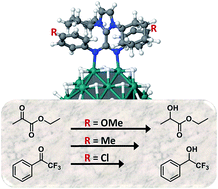
Ligand control of metal nanoparticles (MNPs) is rapidly gaining importance as ligands can stabilize the MNPs and regulate their catalytic properties. Herein we report the first example of Pt NPs ligated by imidazolium-amidinate ligands that bind strongly through the amidinate anion to the platinum surface atoms. The binding was established by 15N NMR spectroscopy, a precedent for nitrogen ligands on MNPs, and XPS. Both monodentate and bidentate coordination modes were found. DFT showed a high bonding energy of up to −48 kcal mol−1 for bidentate bonding to two adjacent metal atoms, which decreased to −28 ± 4 kcal mol−1 for monodentate bonding in the absence of impediments by other ligands. While the surface is densely covered with ligands, both IR and 13C MAS NMR spectra proved the adsorption of CO on the surface and thus the availability of sites for catalysis. A particle size dependent Knight shift was observed in the 13C MAS NMR spectra for the atoms that coordinate to the surface, but for small particles, ∼1.2 nm, it almost vanished, as theory for MNPs predicts; this had not been experimentally verified before. The Pt NPs were found to be catalysts for the hydrogenation of ketones and a notable ligand effect was observed in the hydrogenation of electron-poor carbonyl groups. The catalytic activity is influenced by remote electron donor/acceptor groups introduced in the aryl-N-substituents of the amidinates; p-anisyl groups on the ligand gave catalysts several times faster the ligand containing p-chlorophenyl groups.


 Please wait while we load your content...
Please wait while we load your content...