Anion exchange coupled with the reduction and dimerisation of a copper(ii) nitrate complex of tripyridyl dithioether via a single-crystal-to-single-crystal transformation†
Abstract
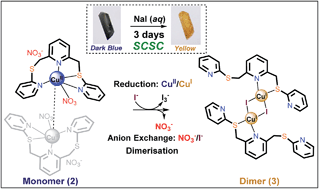
It is a challenge to develop methodologies involving multiple transformations for preparing new materials that cannot be obtained via direct synthesis. Herein, we report an anion exchange process accompanying cation reduction and dimerisation via a single-crystal-to-single-crystal transformation. First, a direct reaction of the flexible tripyridyl dithioether ligand L with CuI afforded a mixture of four bis(ligand) complexes (1a–1d). To avoid the formation of undesired mixed products, a copper(II) nitrate complex-mediated approach involving anion exchange and cation reduction was employed to generate a monomeric complex, [CuII(L)NO3]NO3·toluene (2). When the dark blue crystals of 2 were immersed in an aqueous NaI solution, the crystals were transformed to a pale yellow dimeric copper(I) iodide complex, [(μ-CuI2I2)(L)2] (3). The observed anion exchange promotes the reduction of copper(II) to copper(I) at the expense of I−/I3− oxidation as well as dimerisation via the formation of a Cu2I2 cluster. This result corresponds to the synthesis of a compound that otherwise was not able to be prepared via a direct synthetic procedure.


 Please wait while we load your content...
Please wait while we load your content...