Aggregation-induced emission: mechanistic study of the clusteroluminescence of tetrathienylethene†
Abstract
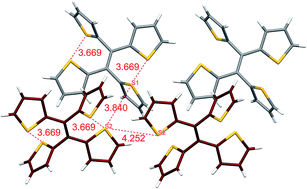
In this work we have investigated the aggregation-induced emission (AIE) behaviour of 1,1,2,2-tetra(thiophen-2-yl)ethene (tetrathienylethene, TTE). The semi-locked and fully-locked derivatives (sl-TTE and fl-TTE) have been synthesized to better understand the mechanism behind the solid state photoluminescence of TTE. TTE is a typical AIEgen and its luminescence can be explained through the mechanistic understanding of the restriction of intramolecular motions (RIM). The emissive behaviour of TTE in the THF/water aggregates and crystal state have also been studied, revealing a remarkable red-shift of 35 nm. A similar red-shift emission of 37 nm from the THF/water aggregates to the crystal state is also observed for (E)-1,2-di(thiophen-2-yl)ethene (trans-dithienylethene, DTE). Crystal analysis has revealed that the emission red-shifts are ascribable to the presence of strong sulfur–sulfur (S⋯S) intra- and intermolecular interactions that are as close as 3.669 Å for TTE and 3.679 Å for DTE. These heteroatom interactions could help explain the photoluminescence of non-conventional luminophores as well as the luminescence of non-conjugated biomacromolecules.


 Please wait while we load your content...
Please wait while we load your content...