Mechanistic insights on the Pd-catalyzed addition of C–X bonds across alkynes – a combined experimental and computational study†
Abstract
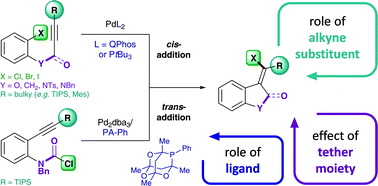
The Pd-catalyzed intramolecular addition of carbamoyl chlorides and aryl halides across alkynes is investigated by means of DFT calculations and mechanistic test experiments. The data suggest a mechanistic pathway that involves oxidative addition, alkyne insertion, cis → trans isomerization and reductive elimination. Our data indicate that oxidative addition is the reactivity limiting step in the addition of aryl chlorides and bromides across alkynes. However, for the corresponding addition of carbamoyl chlorides, alkyne insertion is found to be limiting. Full energetic reaction pathways for the intramolecular additions across alkynes are presented herein and the role of ligands, alkyne substituents and tether moieties are discussed. Notably, the calculations could rationalize a pronounced effect of the alkyne substituent, which accounts for the exceptional reactivity of TIPS-substituted alkynes. In particular, the bulky silyl moiety is shown to significantly destabilize the formed Pd(II)-intermediates, thus facilitating both cis → trans isomerization and reductive elimination, which overall results in a flatter energetic landscape and a therefore increased catalytic efficiency.
- This article is part of the themed collections: Celebrating our 2025 Prizewinners and New reactivity in organic chemistry


 Please wait while we load your content...
Please wait while we load your content...