Organocatalytic, enantioselective synthesis of benzoxaboroles via Wittig/oxa-Michael reaction Cascade of α-formyl boronic acids†
Abstract
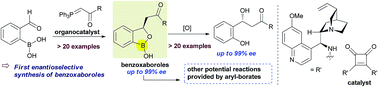
An unprecedented enantioselective synthesis of 3-substituted benzoxaboroles has been developed. An in situ generated ortho-boronic acid containing chalcone provides the chiral benzoxaboroles via an asymmetric oxa-Michael addition of hydroxyl group attached to the boronic acid triggered by the cinchona alkaloid based chiral amino-squaramide catalysts. In general, good yields with good to excellent enantioselectivities (up to 99%) were obtained. The resulting benzoxaboroles were converted to the corresponding chiral β-hydroxy ketones without affecting the enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...