Nature's hydrides: rapid reduction of halocarbons by folate model compounds
Abstract
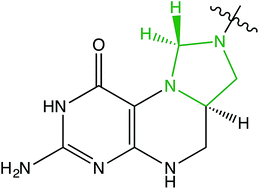
Halocarbons R–X are reduced to hydrocarbons R–H by folate model compounds under biomimetic conditions. The reactions correspond to a halide–hydride exchange with the methylenetetrahydrofolate (MTHF) models acting as hydride donors. The MTHF models are also functional equivalents of dehalohydrogenases but, unlike these enzymes, do not require a metal cofactor. The reactions suggest that halocarbons have the potential to act as endocrinological disruptors of biochemical pathways involving MTHF. As a case in point, we observe the rapid reaction of the MTHF models with the inhalation anaesthetic halothane. The ready synthetic accessibility of the MTHF models as well as their dehalogenation activity in the presence of air and moisture allow for the remediation of toxic, halogenated hydrocarbons.

 Please wait while we load your content...
Please wait while we load your content...