ortho and para chromophores of green fluorescent protein: controlling electron emission and internal conversion†
Abstract
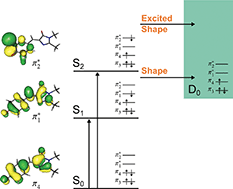
Green fluorescent protein (GFP) continues to play an important role in the biological and biochemical sciences as an efficient fluorescent probe and is also known to undergo light-induced redox transformations. Here, we employ photoelectron spectroscopy and quantum chemistry calculations to investigate how the phenoxide moiety controls the competition between electron emission and internal conversion in the isolated GFP chromophore anion, following photoexcitation with ultraviolet light in the range 400–230 nm. We find that moving the phenoxide group from the para position to the ortho position enhances internal conversion back to the ground electronic state but that adding an additional OH group to the para chromophore, at the ortho position, impedes internal conversion. Guided by quantum chemistry calculations, we interpret these observations in terms of torsions around the C–C–C bridge being enhanced by electrostatic repulsions or impeded by the formation of a hydrogen-bonded seven-membered ring. We also find that moving the phenoxide group from the para position to the ortho position reduces the energy required for detachment processes, whereas adding an additional OH group to the para chromophore at the ortho position increases the energy required for detachment processes. These results have potential applications in tuning light-induced redox processes of this biologically and technologically important fluorescent protein.
- This article is part of the themed collection: 7th EuCheMS Chemistry Congress – Molecular frontiers and global challenges


 Please wait while we load your content...
Please wait while we load your content...