Enantioselective allylic alkylation of stereodefined polysubstituted copper enolates as an entry to acyclic quaternary carbon stereocentres†
Abstract
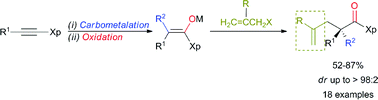
A sequence of regio- and stereoselective carbometalation followed by oxidation of ynamides leads to stereodefined fully substituted enolates that subsequently react with various functionalized allyl bromide reagents to provide the expected products possessing an enantiomerically pure quaternary carbon stereocentre in the α-position to the carbonyl group in excellent yields and enantiomeric ratios after cleavage of the oxazolidinone moiety. Three new bonds are formed in a single-pot operation.


 Please wait while we load your content...
Please wait while we load your content...