Alkene hydrogenation activity of enoate reductases for an environmentally benign biosynthesis of adipic acid†
Abstract
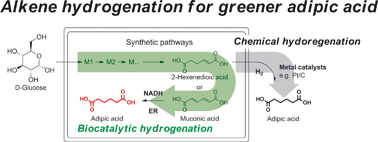
Adipic acid, a precursor for Nylon-6,6 polymer, is one of the most important commodity chemicals, which is currently produced from petroleum. The biosynthesis of adipic acid from glucose still remains challenging due to the absence of biocatalysts required for the hydrogenation of unsaturated six-carbon dicarboxylic acids to adipic acid. Here, we demonstrate the first enzymatic hydrogenation of 2-hexenedioic acid and muconic acid to adipic acid using enoate reductases (ERs). ERs can hydrogenate 2-hexenedioic acid and muconic acid producing adipic acid with a high conversion rate and yield in vivo and in vitro. Purified ERs exhibit a broad substrate spectrum including aromatic and aliphatic 2-enoates and a significant oxygen tolerance. The discovery of the hydrogenation activity of ERs contributes to an understanding of the catalytic mechanism of these poorly characterized enzymes and enables the environmentally benign biosynthesis of adipic acid and other chemicals from renewable resources.
- This article is part of the themed collections: How can chemistry adapt to a low carbon future, Global Energy Challenges: Biofuels and Global Energy Challenges: Fossil Fuels


 Please wait while we load your content...
Please wait while we load your content...