A new reaction route for the synthesis of 2-methyl-5-ethylpyridine
Abstract
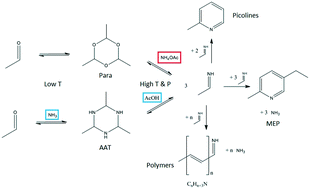
In this work, a novel synthesis route to produce 2-methyl-5-ethylpyridine (MEP) from the cyclic acetaldehyde ammonia trimer (AAT) is explored. The reaction was studied in a semi-batch reactor in the presence of different promoters to adjust the pH of the reaction solution. Among various ammonium salts tested as promoters, ammonium acetate was identified as the most suitable promoter for the reaction. By using a Design of Experiments (DoE) approach, the temperature and concentration of reactants and the promoter were identified as the most important/decisive parameters influencing the course of the reaction. Additional mechanistic investigations were carried out to assess the effect of these parameters in detail and to clarify the by-product formation via oligomer formation.


 Please wait while we load your content...
Please wait while we load your content...