Life cycle assessment of novel supercritical methyl propionate process with carbon dioxide feedstock
Abstract
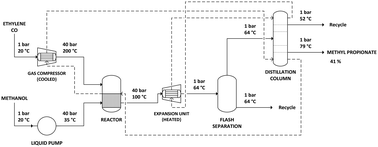
The alkoxycarbonylation reaction can be realized in continuous flow under supercritical conditions by utilizing CO2 as a feedstock instead of CO. Conventionally, the synthesis of the methyl propionate is achieved in the first step of the Lucite Alpha process through the hydroesterification of ethylene with methanol and carbon monoxide. In this paper, synthesis of the methyl propionate process by replacing the carbon monoxide feedstock with CO2 and using a more robust and less expensive catalyst is simulated and evaluated from the perspective of environmental influence. A life cycle assessment was done of the methyl propionate production via the supercritical process utilizing CO2 as feedstock. For all nine impact categories – AP, GWP, EP, FAETP, HTP, Land use, MAETP, ODP and CED –, the novel process was compared to the performance of the existing state-of-the-art carbon monoxide-based process, the Lucite Alpha process. An 80% impact reduction was found for both the Global Warming Potential and the Ozone Depletion Potential. The major contribution to the impact reduction stems from the change from CO to CO2 as a feedstock, since the impact from CO as feedstock is strongly negative while the impact from CO2 as feedstock is strongly positive. Yet, also the supercritical conditions themselves show a notable environmental benefit, besides providing the enabling function for the new chemistry. A remarkable effect on steam, electricity, and cooling energy is given. The higher pressure required for the supercritical CO2 process was found to have minimal effect on the electricity use for compression.

 Please wait while we load your content...
Please wait while we load your content...