Reaction engineering of biocatalytic (S)-naproxen synthesis integrating in-line process monitoring by Raman spectroscopy†
Abstract
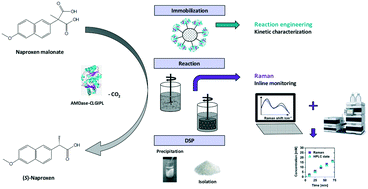
Biocatalytic (S)-naproxen synthesis using an (S)-selective arylmalonate decarboxylase mutant (AMDase G74C/M159L/C188G/V43I/A125P/V156L, AMDase-CLGIPL) exposes a promising environmentally friendly alternative to conventional chemical synthesis strategies. The reaction progress of naproxen synthesis catalyzed by AMDase-CLGIPL covalently immobilized onto a robust acrylate carrier was investigated with respect to reaction engineering. Kinetic characterization of the immobilized enzyme reveals a KM value of 22.1 ± 0.1 mM in the naproxen malonate conversion and an inhibiting effect of the produced naproxen with a Ki of 26.3 ± 1.4 mM. However, an effective process can be realized without in situ product removal yielding (S)-naproxen with an ee of 99%. By optimizing the product work-up, an isolated yield of 92% was achieved with total turnover numbers between 83 000 and 107 000 in five repetitive batches. Furthermore, process monitoring with in-line Raman spectroscopy was successfully applied to analyze the reaction progress with a root mean square error of prediction of 0.8 mM (corresponding to 4%).


 Please wait while we load your content...
Please wait while we load your content...